Plasma – Plastic/Composite Carbon – UV Cured Powder Coating
The Waterborne Symposium, New Orleans LA Feb 24-Mar 1 2019
Michael Knoblauch, President Keyland Polymer UV Powder
Abstract
Plasma treatment is used to modify and improve the surface conditions of various materials. The use of plasma technology on plastic and composite carbon substrates enables these heat-sensitive materials to be successfully coated with ultraviolet (UV) curable powder coatings. Plasma treatment of a part raises its surface energy and removes contaminants. The increase in surface energy enhances the level of surface wetting, which correlates to improved coating adhesion. There are various conditions and processing variables that can be manipulated to increase the effectiveness of plasma treatment of a material surface. A study was conducted on six plastic and composite substrates. The effectiveness of plasma treatment was first determined from the change in surface energy. In general, the surface energy of all samples increased from 30 – 48 dyne/cm to 64 – 70+ dyne/cm. To further study the effectiveness of plasma treatment, the treated plastics and composites were finished with a UV cured powder coating. UV cured powder coatings use less heat energy and cure faster than conventional powder coatings, and do not degrade or deform heat-sensitive substrates. After cure, coating adhesion to the substrate was measured and evaluated. Consistent with the increase in surface energy, improved adhesion was noted on all the test samples.
Introduction
Testing adhesion is the first measurable test when evaluating performance of a coating material, regardless of the type substrate; metal, wood, plastic, composite, paper, glass, or engineered materials. In lay terms, this can be expressed as “does it stick to the substrate”, and if so, what is the measure of adhesion? The measurement of adhesion applies to all forms of coatings; liquids, powder coatings, varnishes, and inks. A coating’s curing system or mechanism is independent of the measure of adhesion. Regardless of the type of curing; ambient air, thermal energy, or radiation energy (UV or electron beam (EB)), the coating has to “stick” to the substrate. A coating that is applied and cured correctly does not guarantee the coating will adhere to the substrate. Proper preparation of the substrate prior to coating is the most important and determinate factor in achieving the desired measure of adhesion for any coating on any substrate.
Preparing a substrate requires an understanding of the physical properties of the substrate, knowledge of the end use of the coated product, followed by identification, selection, and use of the right pretreatment materials. Matching pretreatment materials and processes with the appropriate and desired coating materials assures the desired measure of adhesion is achieved after application and cure. Preparation or pretreatment of ferrous metals is well known; iron phosphate, zinc phosphate, and zirconium pretreatment materials are ubiquitous, and these materials are compatible with many liquid and powder coatings. For most common industrial metal and wood substrates, there exists a broad platform of pretreatment materials and processes corresponding with coating materials that work well and comprise the mature general coatings market.
Development and use of plastics and composites as replacements for metals to achieve weight reductions, fuel savings, product strength, and design objectives are new and exciting opportunities for the coatings market. Coating adhesion is dependent upon preparation and/or pretreatment before coating. A UV cured powder coating was selected for this study because it requires a minimal amount heat to melt the coating (typically not more than 130 C), and the time to melt the powder is one to two minutes followed by near instant UV curing. The amount of heat does not compromise the integrity of the substrates tested. This paper reports the results of an investigation of the use of plasma as a surface modifier on plastic and composite substrates to increase surface energy, and improve the adhesion of a UV cured powder coating.
Measure of Adhesion
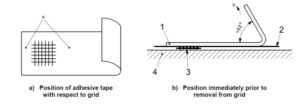
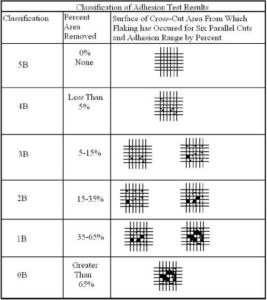
ASTM D33591 is the standard and accepted method to measure and classify adhesion of a coating material to a substrate. The coating is cross-cut scribed at specified spacing using a knife or a cutting device (Figure 1). A pressure sensitive tape is applied over the scribed area and then pulled sharply off the substrate. Adhesion is classified by the percentage of paint removed by the tape from the substrate (Figure 2). 5B is the optimum classification, 4B is often acceptable, anything less is a failure and not acceptable. This classification method is used throughout this study.
Figure 1. Tape application and removal for adhesion test.
Figure 2. ASTM D3359 adhesion classification.
Plasma Treatment
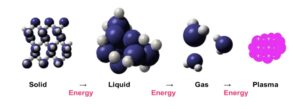
Plasma is often referred to as the fourth state of matter; solid, liquid, and gas being the primary three (Figure 3). Thermal energy, its addition or removal, is the intervening input that changes the nature of matter. Plasma is a gas that has the capacity to conduct electricity. It is the electric energy in combination with the plasma gas that changes and modifies the surface of the treated substrate and influences a coating’s adhesion to the substrate.
Figure 3. States of matter.
A plasma’s interaction with the surface is both physical and chemical. Typically, low energy surfaces (like most plastics and composites) are hydrophobic in nature and manifest a low degree of wettability. Wettability refers to the interaction between a fluid and solid phase, and measures the tendency of a material to spread and flow on a solid surface. Plasma treatment converts a low energy surface to a high energy surface, and makes it more hydrophilic and wettable. Adhesion of a coating has a direct correlation to wettability, the more wettable a surface the better the adhesion.
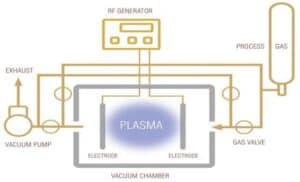
Plasma has six components; electrons, ions, free radicals, byproducts, photons, and neutrals. The two key components are ions, which stimulate physical changes on the material surface, and free radicals, which stimulate chemical changes on the material surface. The ions and free radicals interact with each other through manipulation of the plasma unit’s radio frequency (RF) generator, and gas generator, typically oxygen, argon, or air (Figure 4). The electrical charge and gas interactions are controlled by processing time, RF power, and vacuum pressure. By adjusting these parameters, a successful treatment can be designed for the specific chemical makeup of the substrate being treated. The objective of plasma treatment is to physically clean and etch the surface of a material and provide chemically active bonding sites for coatings to anchor to. The result is a hydrophilic, wettable surface conducive to coating adhesion.
Figure 4. Vacuum plasma unit.3
Atmospheric plasma is a form of plasma treatment. This technology uses a gun or wand as the plasma dispenser and the discharged plasma is targeted onto the surface, impinging an area only as large as the discharge field. Unlike traditional atmospheric plasma treatment systems, vacuum chamber plasma units provide a complete treatment of all surfaces of three-dimensional objects simultaneously. Vacuum plasma has several distinct advantages over atmospheric plasma. As it is not restricted to line-of-sight, the plasma “gets to” all surfaces; ambient conditions, and/or operator variability do not affect the process outcome, time and vacuum pressure is process controlled, and multiple parts can be processed simultaneously.
Vacuum plasma was selected for this study. To process parts using this technology, products are placed inside the treatment chamber, air is evacuated producing a vacuum, the selected gas is injected into the unit, and RF energy is supplied across the electrodes producing the plasma that impinges equally on all the surfaces of the product.
A dyne test was used to measure the surface energy of the treated material. A dyne solution kit consists of various solutions characterized by incremental changes in their dyne/cm values, generally from 30 – 70 dyne/cm. Starting at a low dyne value solution, samples are rubbed onto the surface being tested, and the time taken for the solution to bead on the substrate is noted. Solutions of increasing dyne value are tested incrementally to determine the solution that will bead in approximately two seconds after application, and its dyne value demonstrates the surface energy of the substrate. Higher the dyne value, greater is the surface energy of the substrate.
UV Curable Powder Coatings
The primary types of curing systems are ambient air drying, thermal energy, UV light energy, and, electron beam energy. UV curing is very different from “air dry” or thermal (heat) energy curing systems. The extent of cure is reflected in the number of the crosslinked oligomer chains or fully reacted double bonds remaining in the coating matrix following exposure to the curing system.
UV light has been used to cure inks and coatings for more than 30 years. UV cured liquid materials, i.e., pigmented paints, clear topcoats, pigmented inks, and clear overlay varnishes, dominate the UV coating materials market. Companies have successfully been using UV cured powder coatings for more than 20 years. UV cured powder coatings can replace solvent liquid coatings, thermoset powder coatings, and are a finishing material of interest for many new materials and products. High power UV lamps are the source of UV curing light energy. UV arc and UV medium pressure lamps dominate the market. In the past five years, lamp manufacturers have introduced UV LED lamps and have increased UV energy output. UV LED lamps consume significantly less energy than UV arc and UV medium pressure lamps, do not emit IR energy, have a significantly longer operating life, and a lower total operating cost.
Differential scanning calorimetry (DSC) is a reliable and repeatable method to evaluate the cure of a coating system. Users will often employ methyl ethyl ketone or other solvent for tests to evaluate cure. Research shows that solvent tests to evaluate cure are subjective and can produce false negative and false positive results.
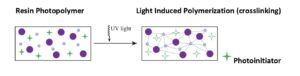
UV curable liquids and powder coatings are photopolymerizable materials with a chemical photoinitiator that instantly responds to UV light energy by initiating the reaction leading to crosslinking (Figure 5). To cure a UV powder coating, a separate melt stage precedes the cure stage. Melting typically takes one to two minutes. UV curing is nearly instantaneous. When considering UV cured coatings, it is necessary to balance the operating parameters of the coating system, process speed, and the coating material, with the spectral range of the UV bulb, and energy output of the lamp system. If the UV lamp’s spectral output does not correspond to the absorption wavelengths of the photoinitiator, or the lamp system is under powered, then the coating may not completely cure.
Resin Photopolymer Light Induced Polymerization (crosslinking)
Photoinitiator
Figure 5. Photopolymerization.
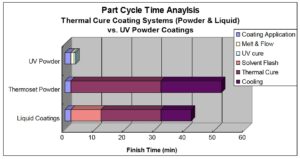
UV cured powder coatings offer many operational benefits: lower energy consumption, the application system has a smaller plant footprint, and increased productivity. In addition to these operational benefits, UV cured powder coatings also have health, safety, and regulatory benefits. Being 100% solids, they are solvent and water-free, and do not require permits to make or use. Figure 6 illustrates the productivity benefit of UV cured powder coating compared to thermoset powder and liquid coatings. Each bar is the sum of time needed for material application and cure.
Figure 6. Part cycle time analysis.
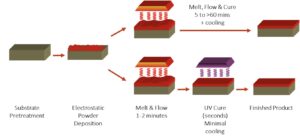
UV cured powder coatings and thermally cured powder coating are similar. The difference is the resin that is specifically formulated to be cured with UV light and the use of photoinitators, as the curing catalysts. Typical resin chemistries include polyesters, epoxies, hybrids, and urethanes. Additives and pigments along with the photoinitiator are added to the resin and complete the formulation. The unique and differentiating characteristic between thermal and UV cured powder coatings is the separation of the melt to cure phase into a short melt phase followed by a near instant UV cure phase (Figure 7).
Figure 7. Melt, flow, and cure.
UV Light Curing Technology
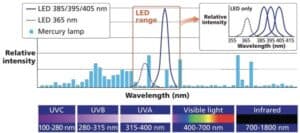
Figure 8 shows the UV to infrared (IR) light spectrum; UVC, UVB, and UVA are the three UV wavelength bands between 100 and 400 nm. Lamp manufacturers named a fourth band, UVV, at 400 to 450 nm. This band is important because the UV energy produced in this band cures thick coatings like UV cured powder coatings. Arc and medium pressure UV lamps broadcast light energy across all the UV bands, and into visible light above 400 nm. Depending upon the type of UV lamp, the energy irradiance and dosage will vary across the UV bands. Photoinitiators absorbs UV light at different wavelengths. The UV light’s emission wave lengths must match the absorbing wave lengths of the photoinitiators to start and complete the cure phase in the coating application process.
Figure 8. Light spectrum.
UV lamp output is described as irradiance (peak intensity). Lamp power (mW/cm2) is measured at a specific distance. The second descriptor is dosage (energy density, mJ/cm2), which is the amount of power reaching the surface of the object being cured as it moves though the lamp’s light field. The higher the dosage, the greater the amount of UV energy to cure the coating. As the distance and line speed varies, the dosage of UV light energy received at the part surface varies. The closer the UV light to the part, the higher the energy dosage to cure the coating. It is important to understand how curing conditions change as lamp power, distance, and time change.
Table 1 shows the UV output measurements of three lamp types; microwave, arc, and UV LED. Distance and speed are constant and measured across the different wavelengths. The UV LED 395 reading is taken at the 395 nm wavelength.
Results and Discussion
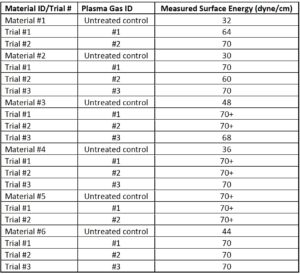
Table 2 reports the surface tension in dyne values (dyne/cm) of six materials; an untreated control followed by trials using different processing conditions. The plasma treating unit used was a Nordson/MARCH AP-1500. Except for the material and processing gas, other conditions were kept constant, i.e., a) position in the unit, b) chamber base pressure, measured in mTorr, c) gas pressure, measured in cc/min, d) unit powder, measured in Watts, and e) plasma treatment time, measured in seconds.